What is a LIMS?
LIMS is the acronym for a Laboratory Information Management System (LIMS).
Overview
LIMS is a system that allows laboratories to trace and track all samples or specimens that are received into the laboratory, tests performed; methods utilized, their results, any changes to results, control limits, QC values, and should also track the analyst, date, and time of each step in the analysis process. Many LIMS systems also have integrated billing, personnel training record tracking, instrument calibration, chemical inventory, and a customer relationship management module. LIMS systems can be quite simple, from a set of Excel templates and Word report templates, to a sophisticated data management application with a secure SQL database solution that provides all of the above features and much more in terms of automated data import from instrument integration, automated e-mailing, faxing of reports, Intelligent Character Recognition (ICR) to scan documents directly into the database, and a secure web-based sample or specimen submission and reporting portal.
LIMS FAQs
There are many factors to consider when selecting a LIMS, although the task may seem daunting with a structured approach you can quickly determine the LIMS that will work best for your laboratory. The key is to invest time to clearly define your data management needs, your total budget including your internal resources, goals, and the role that the LIMS will play in your organization. You may also choose to conduct an internal needs assessment to formalize where the laboratory is currently in terms of automation and where they would like to be. Compare LIMS systems to the specifications that you have prepared and view demonstrations of various products, look at the vendor’s certifications, years in business, their reputation, speak with clients and view the software technology against your expertise. Once you have completed this process you can be sure that the LIMS you select will meet your laboratories challenges both today and into the future.
To begin evaluating your laboratory’s needs it is often best to begin by preparing an outline of current needs, immediate goals and gradually including more and more detail. Begin by understanding the immediate, intermediate and long-term goals of the laboratory (It is often a good idea to interview other LIMS stakeholders) to ensure that their goals are also included. Other stakeholders typically include; the IT manager, the financial/billing manager, and other laboratory division managers. Here are a few questions that you will want to answer:
- Are we strictly an internal support laboratory or do we plan to market our services to external clients in the future?
- How will we get our static table data into the new system? How much will the vendor charge for this?
- Will our LIMS need to integrate with any of our existing enterprise systems?
- Can we leverage any of our existing infrastructures (hardware, software, and expertise) with our new LIMS?
- What regulatory requirements are critical to our certification and must be addressed by the LIMS?
These are just a small sampling of questions. Each laboratory will need to develop their own set of questions and requirements.
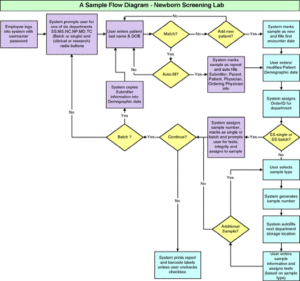
An excellent starting point in defining the laboratory flow and associated needs is to utilize Visio software or some other flow charting tool to create a flow diagram of the current sample or specimen flow through the laboratory. Review your laboratory processes, how orders are received into the laboratory, how samples and specimens are received, accessioned, prepared, tested, quality controlled, validated, approved and finally reported and invoiced to clients? Examine each step in the cycle and try to determine the average amount of time the sample spends at each stage of processing. This will only be a best estimate; once a LIMS is implemented you will have the tools to determine the exact process cycle time for each sample, often referred to as the sample’s turnaround time. The example shown below is a diagram from a newborn screening laboratory.
LIMS Best Practices
Flexible is probably the most overused word in the LIMS industry. And flexible has different meanings to different commercially available packages. Some interpret this to mean the ability of the software to be configurable to different industries, for others it can mean that it can conform to the workflow of your laboratory (some vendors provide a tool set for clients to create their own LIMS), or the ability to model the software to each client’s needs through custom programming. Beware of extensive configuration and custom programming as this can cause the implementation phase to stretch to years versus months and significantly increase total project costs.
ATL’s definition of flexible includes user definable screen captions, custom colors in alerts, limits, program skin, configurability in that clients define the laboratory flow by setting the departments that the samples will flow through, ability to easily create new workflows as business rules change with software written in an object orientated programming language. A user friendly report writer is also critical, so that manager and super-users do not have to be programmers to create simple, yet powerful reports. This is critical, because it is not always easy to anticipate what the laboratory may need in 5-10 years. For this reason select a LIMS vendor that has commitment to continually invest in Research and Development and has provided regular product upgrades. The laboratory is a very dynamic environment and change is the one constant that you can count on, therefore a system that can meet these challenges is critical.
The primary product of the laboratory is information (result data), and the quicker that the laboratory can deliver quality, accurate results the faster that decisions can be made based on that data. Providing clients the ability to retrieve data in real time, over a secure Internet connection, providing results in pdf (read only) format and the ability to automatically email or fax data provides clients rapid access to results. A LIMS allows clients to access this information quickly and effectively.
Basic features fundamental to a LIMS
There is core functionality that is integral to a good LIMS. This includes sample tracking, integrated bar-coding (1D and 2D), sophisticated query searching, integrated QC functions, instrument integration, full audit trail, and enterprise system integration. A sophisticated LIMS system will have many features that the laboratory may not use immediately, but may require as the laboratory grows and utilizes additional automation features. Below are a few of the core functions to evaluate:
- Sample Tracking (invoicing/quoting/chain of custody)
- Data Entry
- Sample Scheduling (stability/shelf life studies)
- QA/QC (integrated)
- Electronic Data Entry (instrument integration)
- Chemical/Reagent Inventory
- Personnel and Equipment Management
- Customer Relationship Management
- Maintenance
Integration with Enterprise Systems
It is important to select a LIMS that is built on industry standard tools. A LIMS system that cannot exchange data with other applications in the laboratory can greatly impair the LIMS utility. Integration with billing systems will eliminate transcription errors and increase productivity. Integration with other systems allows the entire organization to share data and information.
Defining Hardware and Operating Systems
Typically the organization has previously defined the long term hardware and operating system for the laboratory. It is often important to be able to interface with enterprise systems such as accounting, SAP, ERP, Dynamics, Great Plains, JD Edwards, Medicaid billing, etc.
There are many different platforms, many older text-based systems utilize the VAX, UNIX, or AS400 are being replaced with modern systems have a graphical user interface such as Windows 8. The database market leaders are Microsoft SQL Server and Oracle for the SQL (Structured Query Language) database engines.
Following the development of specifications and comparing system functionality, the next step is to compare the relative value of the various quotes. Do the quotes include the total cost of system ownership, hardware costs, software costs, user fees, installation, training, and annual support costs? Be sure to inquire about hidden costs, such as product upgrades, custom programming or purchasing additional user licenses. Request a quote that will capture the complete system costs. It is important to flesh out all system costs upfront. A system that starts out costing $135,000 may end up costing the laboratory much more ($195,000) than an inclusive system that costs $150,000.
Ready to Get Started?
We’re here to support all of your LIMS needs!